
干细胞是一类具有自我复制和多向分化潜能的原始细胞。它们存在于伴侣动物的许多组织中,能够发育成各种不同类型的细胞,用于修复和再生受损的组织和器官。
干细胞疗法使用伴侣动物自身的干细胞进行治疗,即自体干细胞疗法,利用自身干细胞的再生能力,指导它们治疗由创伤和衰老引起的疾病。
干细胞疗法提供了一种定制化且独特的解决方案,将有助于确保您的爱宠未来的健康和生活质量。因细胞来自宠物自身,因此不用担心排斥反应或副作用!
零排异风险:使用伴侣动物自身细胞,无需药物抑制免疫
高活性保障:年轻时期储存的细胞增殖能力更强
即时可用:危急时刻无需等待细胞培养周期
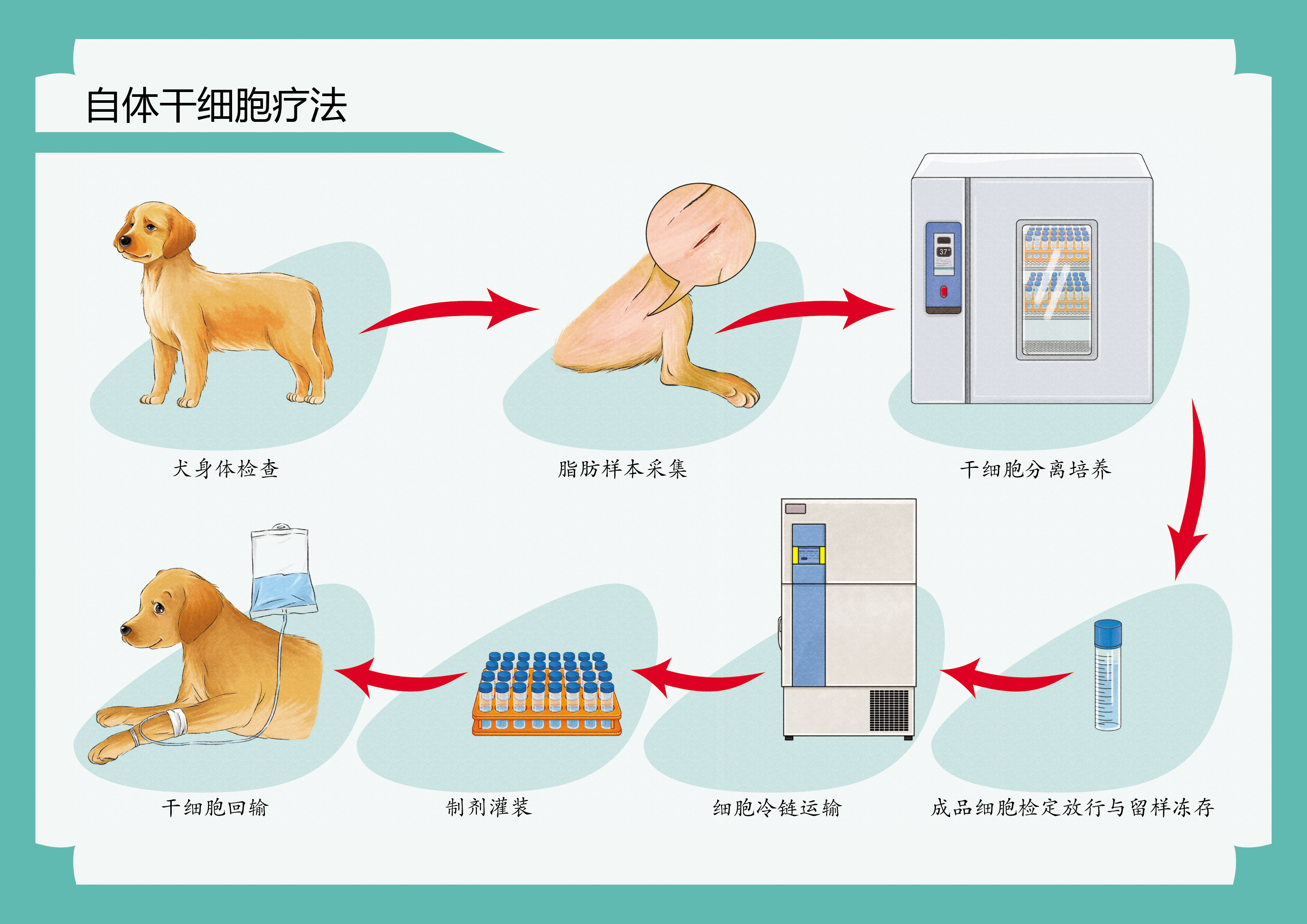
方案一:自体干细胞库
采集来源:新生幼崽脐带/自体皮下脂肪等易获取组织
黄金窗口:建议在宠物3岁前完成首次储存
服务保障:终身冻存+三次免费复苏激活
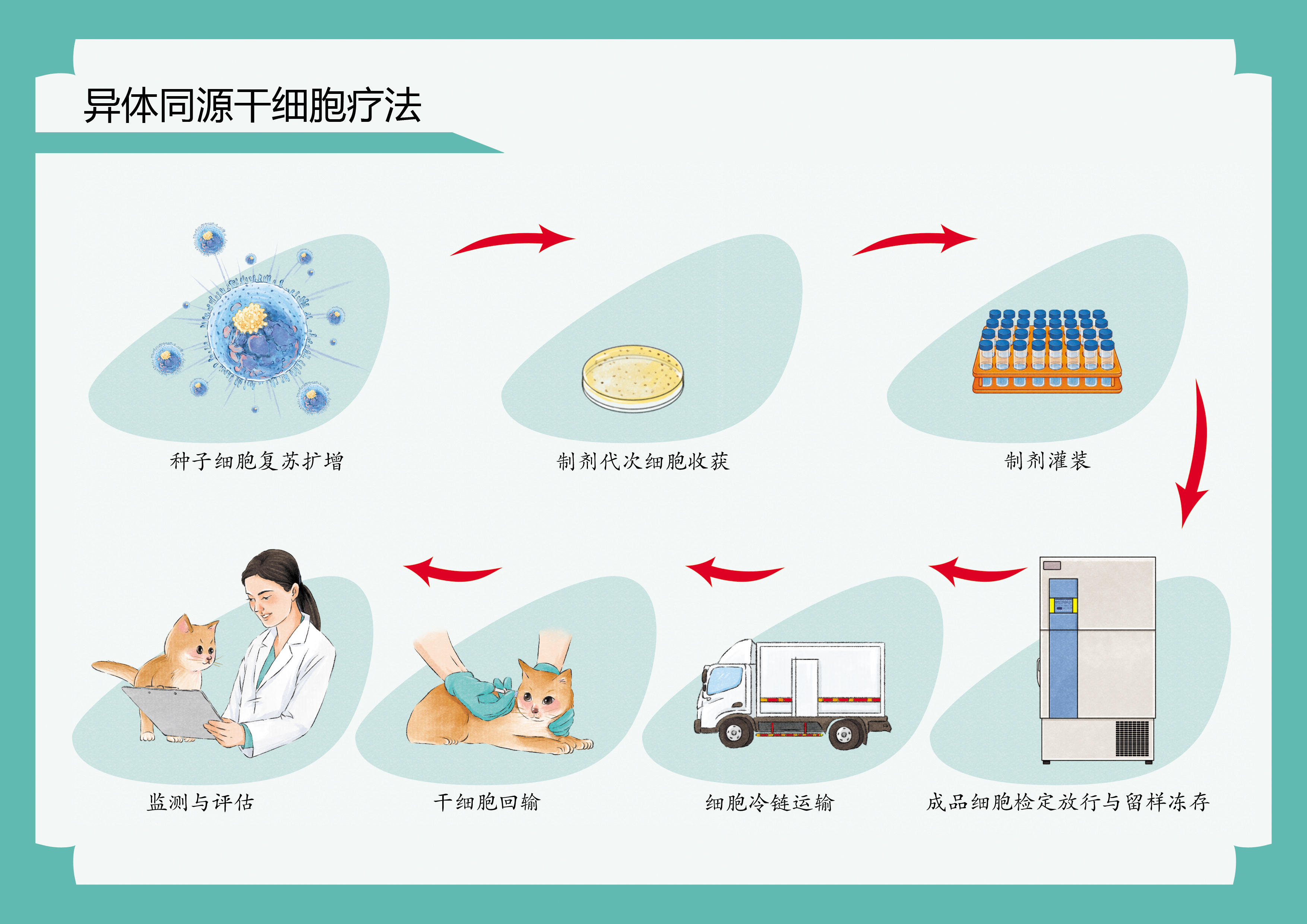
方案二:异体同源细胞库
覆盖品种:约克夏/吉娃娃/拉布拉多/西高地/金毛/哈士奇/藏獒/德文/缅因/紫金渐层/蓝金渐层/布偶/暹罗等常见犬猫品种
应用优势:异体同源干细胞疗法比自体来源的疗法更具成本效益,并且效果相同
特别推荐:适合未储存自体干细胞的紧急治疗
(1)神经系统疾病(脊髓损伤)
(2)消化系统疾病(口炎、扁桃腺炎、炎性肠病、咽炎、肝损伤)
(3)呼吸系统疾病(肺炎、支气管炎)
(4)运动系统疾病(骨折、关节炎、骨髓炎)
(5)内分泌系统疾病(糖尿病、皮肤病)
(6)泌尿系统疾病(肾损伤、膀胱炎、尿道炎)
(7)抗衰老
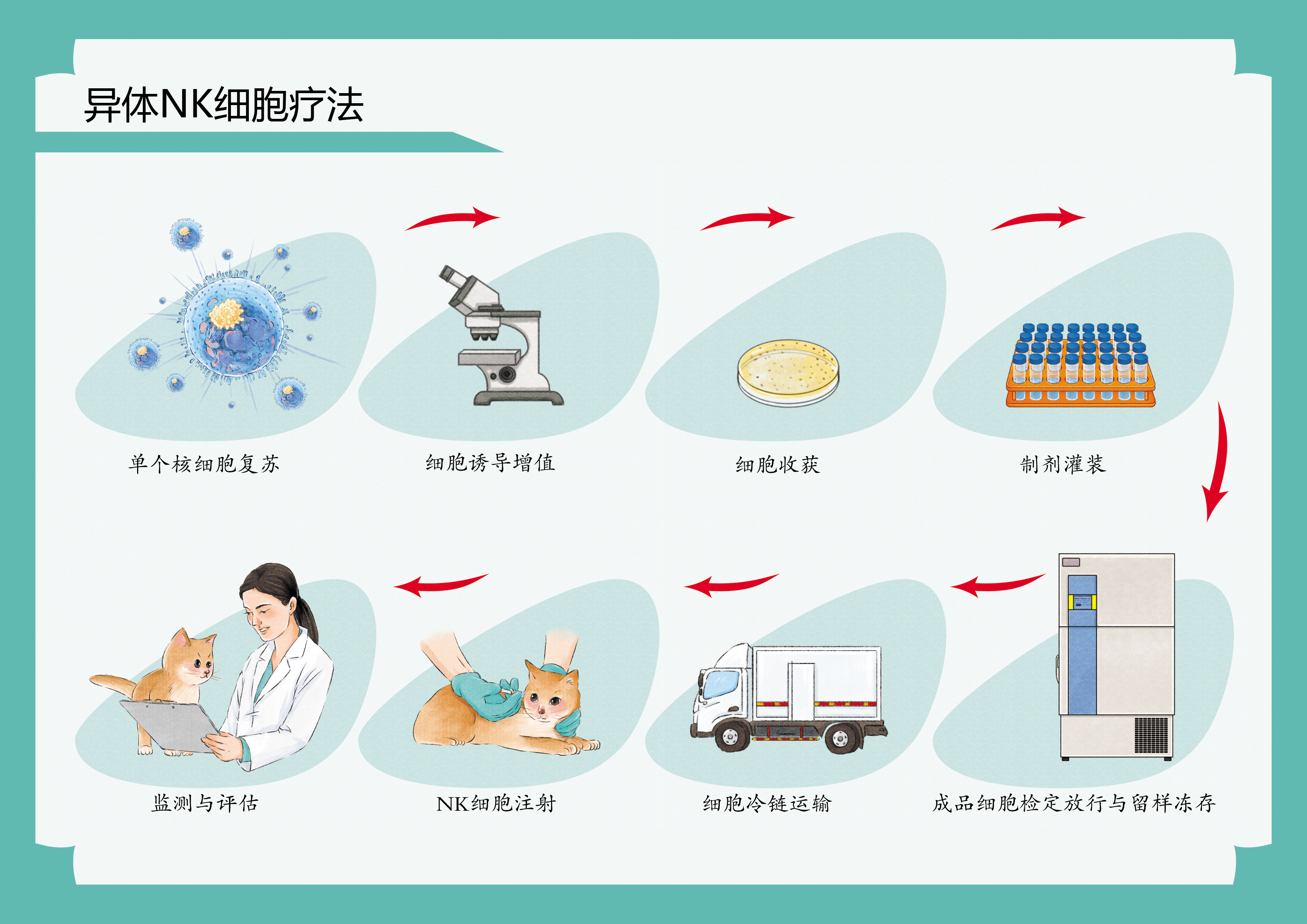
初步评估:我们的专家团队将对宠物进行全面的健康评估,确定干细胞治疗的适应症和方案。 间充质干细胞采集和制备流程:
认识免疫系统的特种部队NK细胞——与生俱来的病毒清道夫
NK细胞疗法是一种利用自然杀伤细胞(Natural Killer Cell,简称NK细胞)来治疗疾病的方法。
NK细胞是免疫系统中的快速反应部队,具备两大核心能力:
精准识别:无需抗体标记即可锁定异常细胞
多重杀伤:通过释放穿孔素/颗粒酶摧毁病变细胞
免疫兼容性:100%匹配宠物自身生物特征
活性优势:年轻时期储存的细胞杀伤效能更强
快速响应:危急时刻可立即启动治疗程序
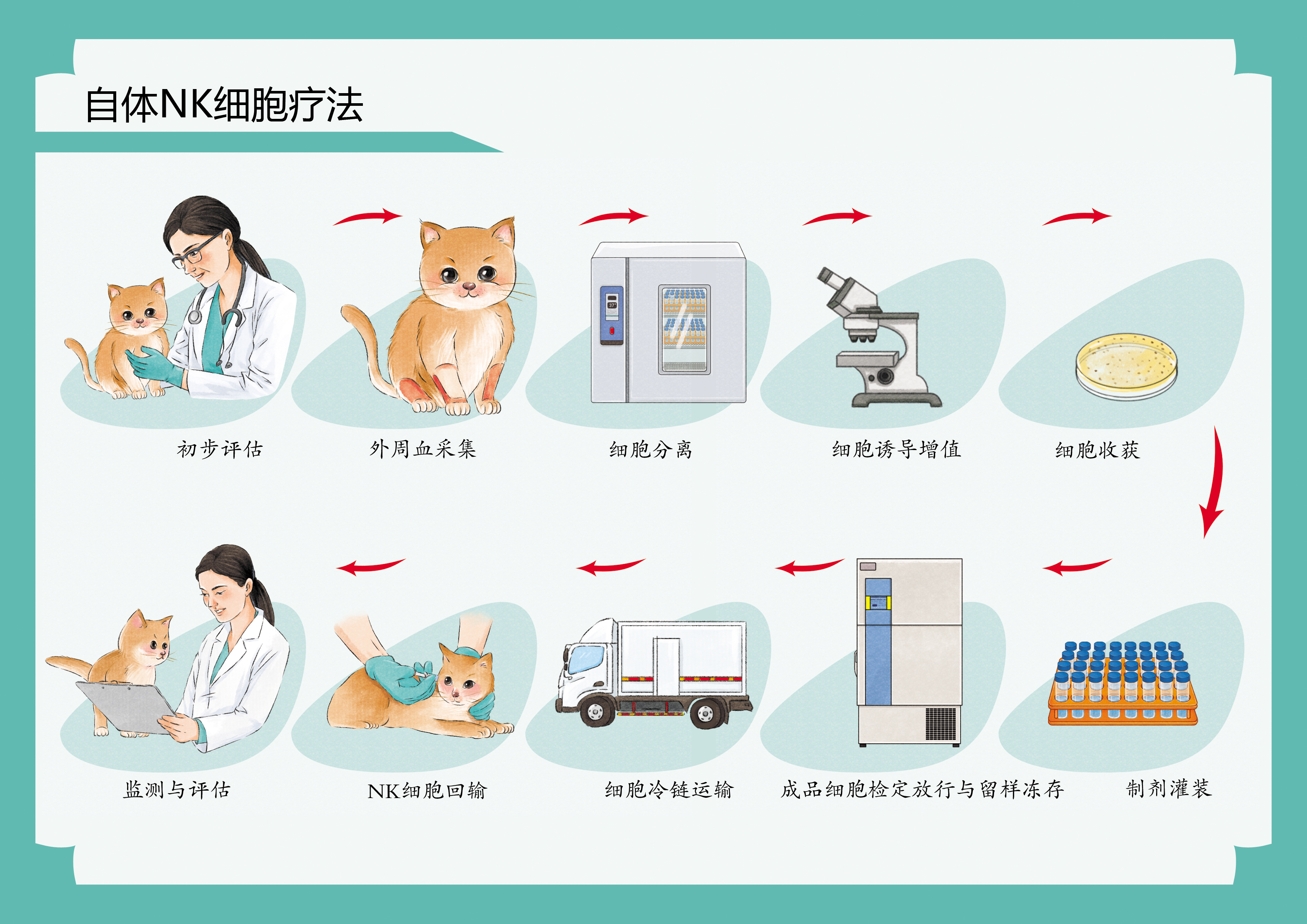
方案一:自体NK细胞库
采集来源:外周血
黄金时期:建议在宠物健康状态良好时储存
服务保障:液氮冻存+活性定期检测
方案二:强化异体细胞库
覆盖病种:肿瘤疾病(淋巴瘤、乳腺癌、黑色素瘤)、病毒感染性疾病(猫泛白细胞减少症、犬瘟热)
应用场景:适合自体细胞活性不足的紧急情况


外泌体——生命体的智能纳米快递
外泌体是细胞分泌的30-150nm囊泡,搭载着天然的修复指令系统:
精准导航:这些外泌体如同细胞的“快递员”,表面标记蛋白锁定受损组织并传递修复信号
信息包投递:携带300+种活性成分(mRNA/生长因子),促进受损组织的再生和恢复
安全无痕:天然载体不触发免疫排斥反应,且能在体内长时间保持活性
穿透力强:纳米尺寸直达深层病变组织
长效修复:持续释放活性成分
多靶点调控:同步实现抗炎+再生+免疫调节
| 治疗维度 | 作用机制 | 临床获益 |
|---|---|---|
| 分子快递 | 递送miRNA修复遗传指令 | 重启细胞正常代谢 |
| 微环境重塑 | 调节细胞因子网络平衡 | 阻断慢性炎症循环 |
| 干细胞联动 | 增强内源性干细胞活性 | 加速组织原位再生 |
(1)皮肤疾病(伤口愈合、过敏性皮炎、皮藓)
(2)眼科疾病:(青光眼、视网膜病变、干眼症、角膜损伤)
(3)口腔疾病:(牙周炎、口腔溃疡、粘膜破损修复)

[1] Nixon A, Dahlgren L. Adipose-Derived Pluripotent Stem Cells for Tendon Repair. Submitted to Equine Vet J, 2006.
[2] Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 2003 Dec;48(12):3464-74.
[3] Nathan S, Das De S, Thambyah A, et al. Cell-based therapy in the repair of osteochondral defects: A novel use for adipose tissue. Tissue Eng 2003 Aug;9(4):733-44.
[4] Awad HA, Butler DL, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng 1999 Jun:5(3):267-77.
[5] Bruder SP, Kraus KH, Goldberg VM, et al. The effects of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am 1998 Jul:80(7):985-96.
[6] Bruder SP, Jaiswal N, Kadiyala S, et al. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res 1998 Oct:355 Suppl:S247-56.
[7] Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, MARCH KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004 Mar 16;109(10):1292-8.
[8] Shin K O, Ha D H, Kim J O, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis[J]. Cells, 2020, 9(3): 680.
[9] Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling[J]. Scientific Reports, 2017, 7(1): 13321.
[10] Wu K Y, Ahmad H, Lin G, et al. Mesenchymal stem cell-derived exosomes in ophthalmology: a comprehensive review[J]. Pharmaceutics, 2023, 15(4): 1167.
[11] Alió del Barrio J L, De la Mata A, De Miguel M P, et al. Corneal regeneration using adipose-derived mesenchymal stem cells[J]. Cells, 2022, 11(16): 2549.
[12] Wu K Y, Ahmad H, Lin G, et al. Mesenchymal stem cell-derived exosomes in ophthalmology: a comprehensive review[J]. Pharmaceutics, 2023, 15(4): 1167.
[13] Qu L, He J, Ge L, et al. Exosomes derived from IFNγ-stimulated mesenchymal stem cells protect photoreceptors in RCS rats by restoring immune homeostasis through tsRNAs[J]. Cell Communication and Signaling, 2024, 22(1): 543.
[14] Garcia-Olmo D, Garcia-Arranz M, et al. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005 Jul;48(7): 1416-23.
[15] Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005 Jun;57(6):874-82.
[16] Harman R, Cowles B, Orava C, et al. A retrospective review of 60 cases of joint injury in sport horses treated with adipose-derived stem and regenerative cell therapy. VetStem internal data, 2006.
[17] Harman R, Cowles B, Orava C., et al. A retrospective review of 52 cases of suspensory ligament injury in sport horses treated with adipose-derived stem and regenerative cell therapy. Presented at the Veterinary Orthopedic Society Convention Mar 3-10, 2007 in Sun Valley, ID.
[18] Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 2004 May;22(5):560-7.
[19] Jeong JH, Ki YW, Kim JY, Jan SH, Kim SH, Chang Y. Adipose Tissue Derived MSC Enhances Motor Function in Rats with Cerebral Infarction. IFATS 2005. Oral Presentation.
[20] Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001; 32: 1005-1011.
[21] Luyten FP. Mesenchymal stem cells in osteoarthritis. Curr Opin Rheumatol 2004 Sep;16(5):599-603.
[22] Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 2005 Sep;54(3):132-41.
[23] Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002 Dec 10;106(24):3009-17.
[24] Herreros J, Prosper F, Perez A, et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J 2003 Nov;24(22):2012-20.
[25] El-Badri NS, Maheshwari A, Sanberg PR. Mesenchymal stem cells in autoimmune disease. Stem Cells Dev 2004 Oct;13(5):463-72.
[26] Pluchino S, Martino G. The therapeutic use of stem cells for myelin repair in autoimmune demyelinating disorders. J Neurol Sci 2005 Jun 15;233(1-2):117-9.
[27] Uccelli A, Zappia E, Benvenuto F, et al. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opin Biol Ther 2006 Jan;6(1):17-22.
[28] Oedayrajsingh M, Breuls R, Schouten T, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev 2007;16:91-104.
[29] Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Throm 2006 Apr;13(2):77-81.
[30] Yanez R, Lamana ML, Garcia-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease (GVHD). Stem Cells Aug. 24, 2006 Online version.